The 2014 Ebola outbreak and the COVID-19 pandemic have exposed the enormous dependence of Africa on imports of medical products. Currently, only 1% of vaccines and 2% of medicines administered in Africa are produced on the continent.1 This dependence on vaccine imports and the resulting delay in COVID-19 vaccine delivery shifted the perception of the medicines sector. Local manufacturing is no longer perceived as a marginal issue of mere economic integration but is now considered a central question of security and autonomy. 2
To increase health coverage and local manufacturing, several strategies emphasize the importance of establishing the African Medicines Agency (AMA) as a continental regulator for medicines.3 In March 2021, the CEO of the International Alliance of Patients' Organizations, Kawaldip Sehmi, "explained that AMA is essential for a Pan-African vision of health in the future […]. COVID-19 has reset the terms of our social contract, that is why we need this agency."4 Accordingly, the establishment of the AMA is one of the fastest integration processes in the history of the African Union.5
The establishment process of the AMA reflects the current drivers and roadblocks of regional integration in Africa, especially in terms of the role of regional hegemons and external donor support. This paper provides background information on the AMA, outlines the framework for regional integration, analyzes the role of regional hegemons and external donors for the establishment of the AMA, and concludes with a critical discussion of the findings. To shed light on the complexities of the agency's establishment process, this paper applies concepts from the analysis of regional integration to the case of the AMA. Specifically, a synthesis report titled "The political economy of regional integration in Africa" by Vanheukelom, Byiers, Bilal and Woolfrey will serve as a framework.6
The African Medicines Agency
The limited capacity of African medicine regulators inhibits access to medicines on the continent and curbs the economic potential of the pharmaceutical sector. For a medical product to reach a patient, a bottleneck is the product's approval by the responsible regulatory agency. In Africa, 54 of the 55 countries have their own National Medicines Regulatory Authority. However, approximately 15% of them have the legal mandate to perform all relevant regulatory functions, and only five have substantial regulatory capacity.8 Accordingly, the Pharmaceutical Manufacturing Plan for Africa emphasizes that "fragmented and weak regulatory systems"9 present a key challenge to production in Africa, thus constraining the growth of the pharmaceutical sector. The African Health Strategy 2016-2030 further highlights that "the regulation of medical products and technologies at the continental level should be prioritized to support availability of quality products."10
Significant steps have been taken to improve the medicine regulatory system in Africa. In 2009, the African Medicines Regulatory Harmonisation Initiative (AMRH) was launched, aiming to harmonize medicine regulation within Regional Economic Communities (RECs) and thus attract more manufacturers to enter the (sub-)regional pharmaceutical markets.11 The initiative has since been implemented in five out of eight RECs.12 In 2016, the African Union (AU) Heads of State and Government endorsed the AU Model Law on Medical Products Regulation, developed by AMRH to further embed harmonization in national legislation.13 Both efforts were meant to serve as a basis for the establishment of the AMA.14
The AMA is an AU agency with the objective of ensuring that "African People have Access to essential Medical Products and Technologies."15 Building on the AMRH and AU Model Law, the AMA Treaty was adopted in 2019 and entered into force in 2021.16 As the continental regulatory agency for medical products, the AMA is tasked with a) coordinating the sub-regional and national regulatory systems, b) assessing selected medical products and conducting the regulatory oversight, and c) facilitating harmonization and cooperation among regulatory agencies in Africa.17 As of January 2024, twenty-six countries have signed and ratified the treaty, nine have only signed it, and twenty countries have done neither.18 Rwanda has been designated as the AMA's host country, but the governing board and director general have yet to be appointed, thus the implementation of the AMA has been stalled.19
Regional Integration in Africa
The establishment of the African Medicines Agency can be seen as a case of regional integration. This paper considers regional integration as a process through which neighboring countries increase their cooperation on specific policy efforts. In line with the distinction of formal and informal integration,20 this paper focuses purely on the formal and institutionalized aspects of regional integration.
In their synthesis report, Byiers et al.21 focuses on the gap between African regional integration policies and their implementation. Based on studies of six regional organizations, the AU, and five sub-regional organizations, the authors explore the role of structural, institutional, actor-related, sectoral, and external factors. Among their ten central findings, the following two serve as a framework for analyzing the drivers and roadblocks to the establishment of the AMA:
- Regional integration is shaped by the interests of regional hegemons. When regional policy efforts align with the hegemon's interests, hegemons often take the lead and provide substantial support. However, if a hegemon's interests do not align, they might exploit their power to undermine integration efforts or instrumentalize them for their own benefit.
- Most regional organizations in Africa heavily depend on external donor support. While this funding can facilitate regional integration efforts, it can also have the opposite effect if poorly managed. This may lead to "empty signaling of reforms by regional organizations, agenda inflation, reduced ownership, and missed opportunities to strengthen institutional functions that are pivotal for the governance of regional organizations."22
Regional Hegemons
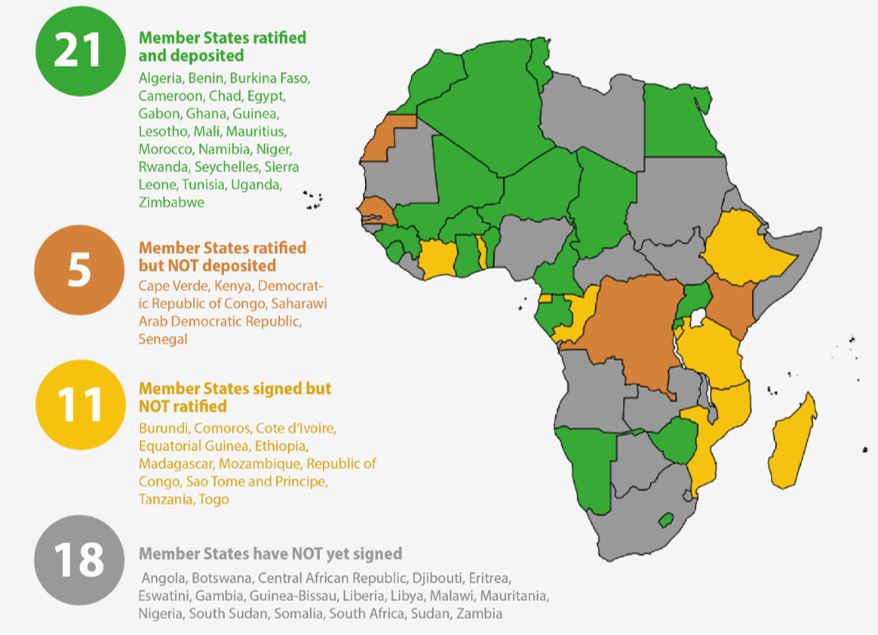
Regional hegemons strongly shape the integration process towards a functional AMA, as illustrated by the process of signing and ratifying the AMA Treaty. This process is fundamental as ratification required fifteen countries signature of the AMA Treaty for it to enter into force. The AMA Treaty reached this threshold in October 2021, more than two years after the treaty had been finalized. AMRH and AMA proponents still advocate for more countries to sign and ratify as every additional country increases the agency's available resources and relevance. Countries are expected to benefit from joining the agency as it improves their ability to assess complex products, inspect manufacturing plants abroad, and conduct other more difficult or costly regulatory functions.23 Nevertheless, many countries have not signed the treaty because they fear losing autonomy and funding sources through centralizing their assessments.24 Increasing attention paid to the AMA also politicized the process, so that overarching issues in domestic politics – rather than purely technocratic reasoning – continue to impact countries' decision to sign and ratify. 25 The importance of this process is further underlined by the fact that Health Policy Watch, a global news outlet on health policy, has a designated AMA countdown tracking the progress made in each country (see graph).26
In the context of African medicine regulation, regional hegemons are defined by their economic power and their regulatory expertise. As in most sectors, the economic size of a country strongly determines its political and economic role within the region. This is reflected in the discourse among health policy makers and experts, who emphasize that "Kenya's signing and ratification is a huge milestone in the journey to regulatory harmonisation being that this is one of the biggest economies in [the] region."27 Malawi health officials explicitly mentioned that Kenya's move was decisive in their decision to sign the treaty.28 On the flip side, an industry representative called the AMA "a big mess" because Nigeria, Ethiopia, and South Africa had not yet ratified the treaty.29 The late ratification in these economically powerful countries poses the risk of confusing manufacturers and consequently delaying vaccine manufacturing on the continent, according to a Nigerian health equity advocate.30 In line with findings by Byiers et al.,31 the economically powerful countries therefore have both: the potential to drive integration as is the case with Kenya, and the capacity to undermine integration efforts as South Africa and Nigeria do.
Figure 1: AMA Countdown by Health Policy Watch, as of 4 January 202432
Arguably, a second factor determining hegemony in medicine regulation is the expertise and capacity of a country's regulatory agency. Of the fifty-four regulatory agencies in Africa, only the authorities in Egypt, Ghana, Nigeria, South Africa, and Tanzania have been classified through WHO benchmarking as having significant regulatory capacity (maturity level 3 out of 4).33 The regional harmonization initiatives in the RECs and the AMA all build on these mature agencies to serve as experts and peer-teachers to less mature agencies. Typical pathways for this are work sharing, assessing products collaboratively in multi-national teams, or practicing regulatory reliance – a process by which regulators do not have to assess each product themselves but invoke the assessment of other agencies. 34
Although this conceptualization of hegemony does not align with Byiers et al.35 in the narrow sense, expanding the definition to include hegemony based on regulatory expertise adds more nuance to the analysis of the AMA's establishment. Among the five regulatory hegemons, only Egypt and Ghana have ratified the AMA treaty so far; Tanzania only signed the treaty while Nigeria and South Africa have done neither.36 The resulting lack of participation by three of the five most mature agencies on the continent challenges the salience of the AMA as a relevant and functional agency. Thus, their regulatory expertise indeed puts these five countries in the position of regional (sector-specific) hegemony in the establishment of the AMA.
Donor Support
The AMA – and AMRH as its predecessor – strongly rely on external donor support. The Business Plan of the AMA foresees that AMA relies on diverse funding sources by combining a) direct contributions from member states as per the financial rules (yet to be developed by Governing Board and State Parties), b) direct contributions from partners, c) revenue generated through service fees (similar to the European Medicines Agency), d) and innovative financing mechanisms like Social Impact Bonds and an Endowment Fund. 37 While these provisions highlight the desire among African policymakers to reduce dependence on donor funding and ensure autonomy and sustainability, the specific targets for 2022 suggest that such independence will not be attained soon. Formulated in 2015, the business plan set out that member state contributions should cover 100% of the operating budget by 2022. Partner contributions should account for 75% of the program budget, with the innovative funding mechanisms covering the remaining 25%.38 These diversification efforts underscore the political attempt to avoid total donor dependency on the operational side and limit it for the entire program. Due to a lack of accessible data on the financial sources of the AMRH and AMA to date, however, these target values cannot be evaluated against de facto developments. Thus, an implementation gap – the key roadblock to regional integration according to Byiers et al.39 – might still occur.
Another mechanism adopted to streamline and control donor engagement in support of regulatory integration in Africa is the AMRH Partnership Platform. Initiated in 2018 by the AMRH Steering Committee, the platform aims to coordinate different stakeholders wishing to contribute to the AMRH and AMA. As of January 2024, more than 40 partners constitute the Partnership Platform, with the Bill & Melinda Gates Foundation and Swissmedic taking the lead. The remaining partners provide financial and/or technical support based on identified needs by various AMRH committees. 40
The rationale for the partnership platform is twofold. First, the platform is meant to facilitate the coordination of the partners to reduce duplicative efforts and improve the efficient use of resources.41 Given the sheer number of stakeholders in the field and the scarcity of resources, this is crucial in streamlining the integration efforts. Second, and potentially more important, the Partnership Platform is an attempt by African policymakers to stay in the driver's seat of the AMA's establishment, despite the many – and partially competing – donor interests.42 By actively managing the partners through the platform, the AMRH secretariat and national experts set the agenda, priorities, and objectives. As such, the AMRH Partnership Platform can be interpreted as a response to the donor-related challenges which Byiers et al.43 outline, principally the fragmentation and management of aid, as well as external agenda distortions.
Conclusion
The establishment of the African Medicines Agency reflects the drivers and roadblocks of regional integration in Africa, especially in terms of the role of regional hegemons and external donor support. Building on Byiers et al., this paper found that firstly, regional hegemons – in economic and regulatory terms – indeed shape the integration process. While Kenya's ratification of the AMA treaty led Malawi to quickly follow suit, the delays from South Africa, Nigeria, Ethiopia, and Tanzania are associated with hesitation in other countries as well as confusion among manufacturers. Secondly, the AMA's business plan and the AMRH Partnership Platform represent attempts by African policy makers to manage donor support and thus limit negative repercussions, such as aid fragmentation and agenda distortions. However, due to the early stage of the AMA establishment and the sensitivity of the issue, no information seems to be publicly available to analyze the implementation and success of these measures. Applying the findings from Byiers et al. to the African Medicines Agency therefore yields substantial insights on the drivers and roadblocks to the agency's establishment.
Due to the limited scope of this paper, further analysis should be directed towards the remaining issues. Most importantly, analyzing the impacts of external funding has been restricted by the lack of data on the finances of AMRH and AMA. While this limits the relevance of section 5 (donor support), it also questions the transparency of the AMA. Close attention should be paid to the funding sources of the AMA in the future, as they reflect member state commitments and donor influences. Further, Byiers et al. point to the relevance of elites' interests for the positioning of their countries, which is particularly important in the case of regional hegemons. Future research should be dedicated to analyzing how different scenarios of local pharmaceutical manufacturing could impact the domestic elites and their support for the AMA. Another relevant aspect which went untouched is the role of individual personalities and leadership in shaping the course of the AMA. From her experience in working with the European and African Medicines Agencies, the author is aware that the establishment of the AMA thus far has been shaped by a few decisive individuals. Conducting research – perhaps through qualitative interviews and observations – on their roles would contribute greatly to understanding the AMA's establishment.
About the Author
Josefine Petrick is a Master in International Affairs student at Johns Hopkins University SAIS from Cologne, Germany. She is passionate about global health and access to medicines and has dedicated her studies and work to the field. She obtained her Bachelor in Liberal Arts and Sciences from Maastricht University and UC Berkeley, with a focus on international relations and public health policy. Her research addressed policies against antimicrobial resistance (AMR) in Spain and Germany, and the work of global health actors such as GAVI and the Global Drug Facility. Before SAIS, Josefine worked as a trainee in the European Medicines Agency's International Affairs Department, which included collaboration with the African Medicines Agency.
Footnotes
Download footnotes here.

